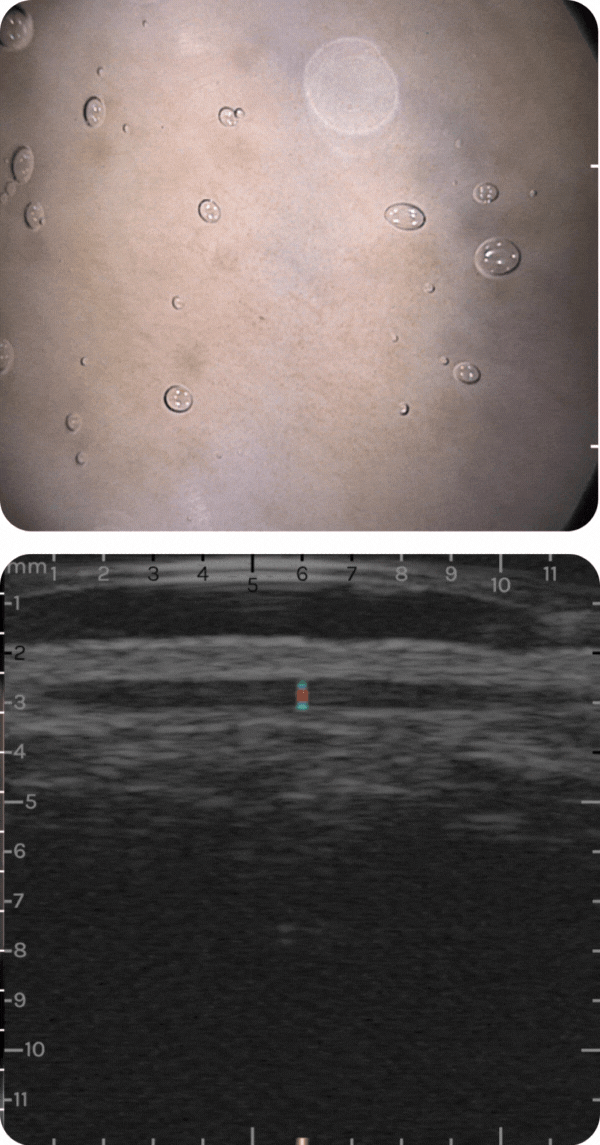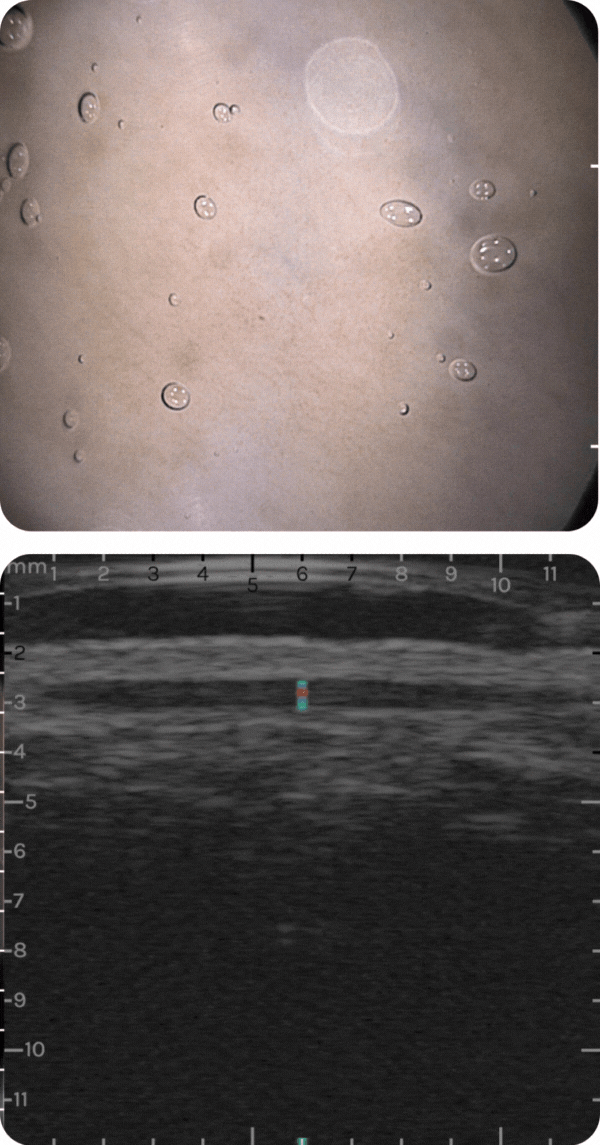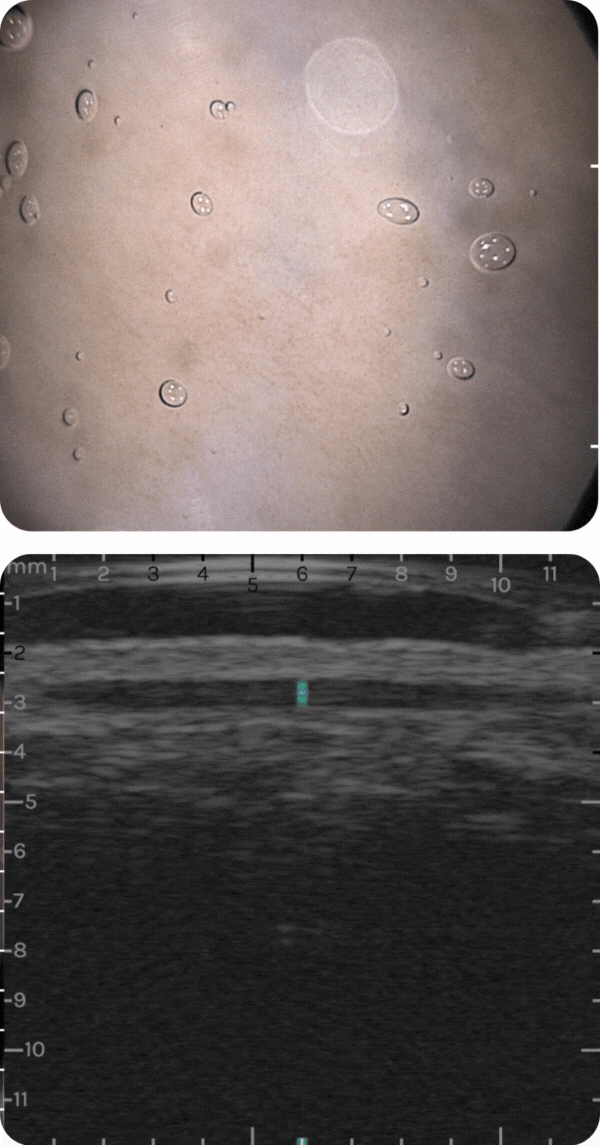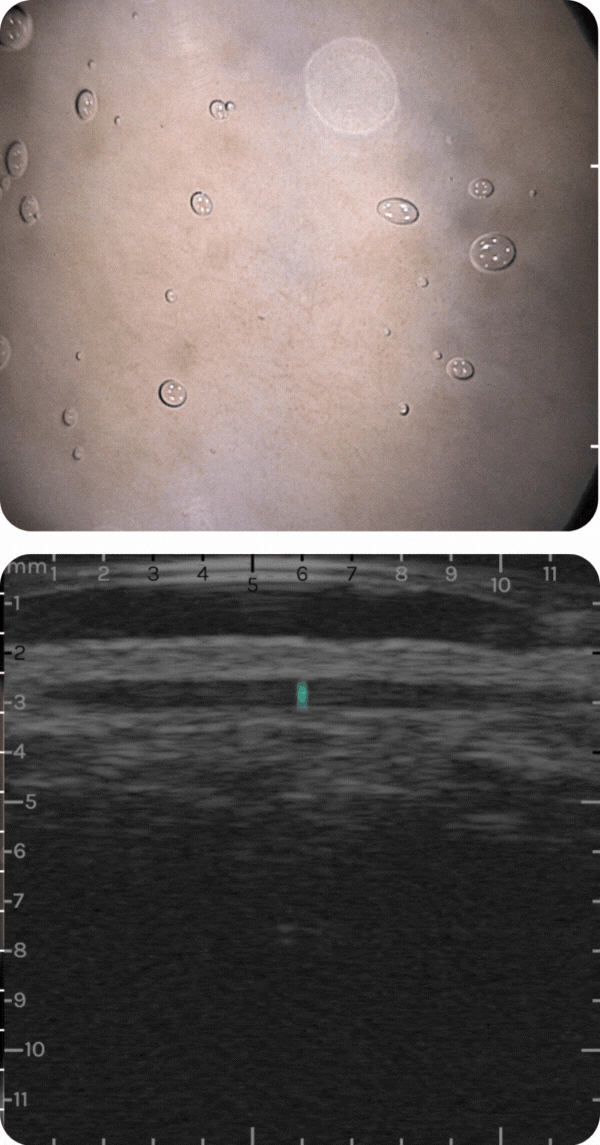
sdfsdfsdf
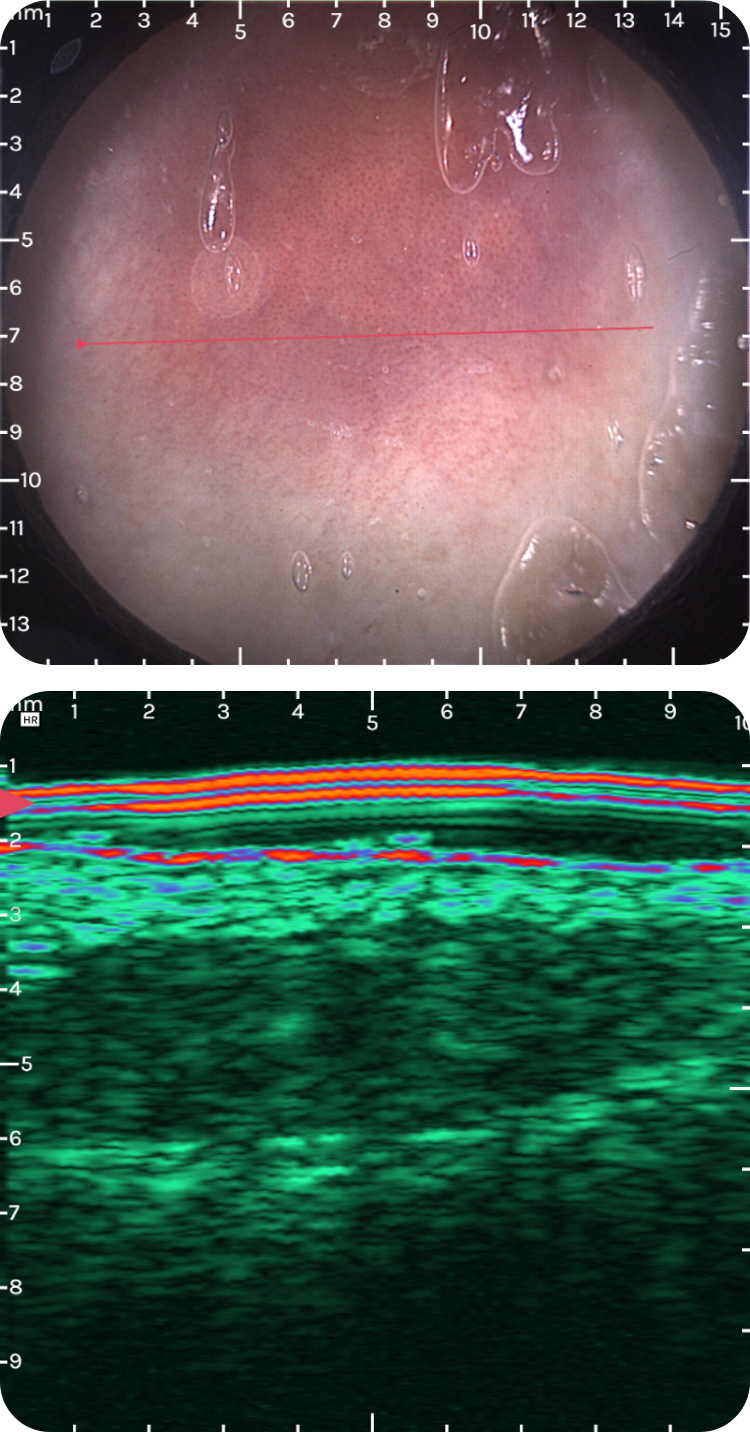
sdsdfsdf

sdfsdfsd
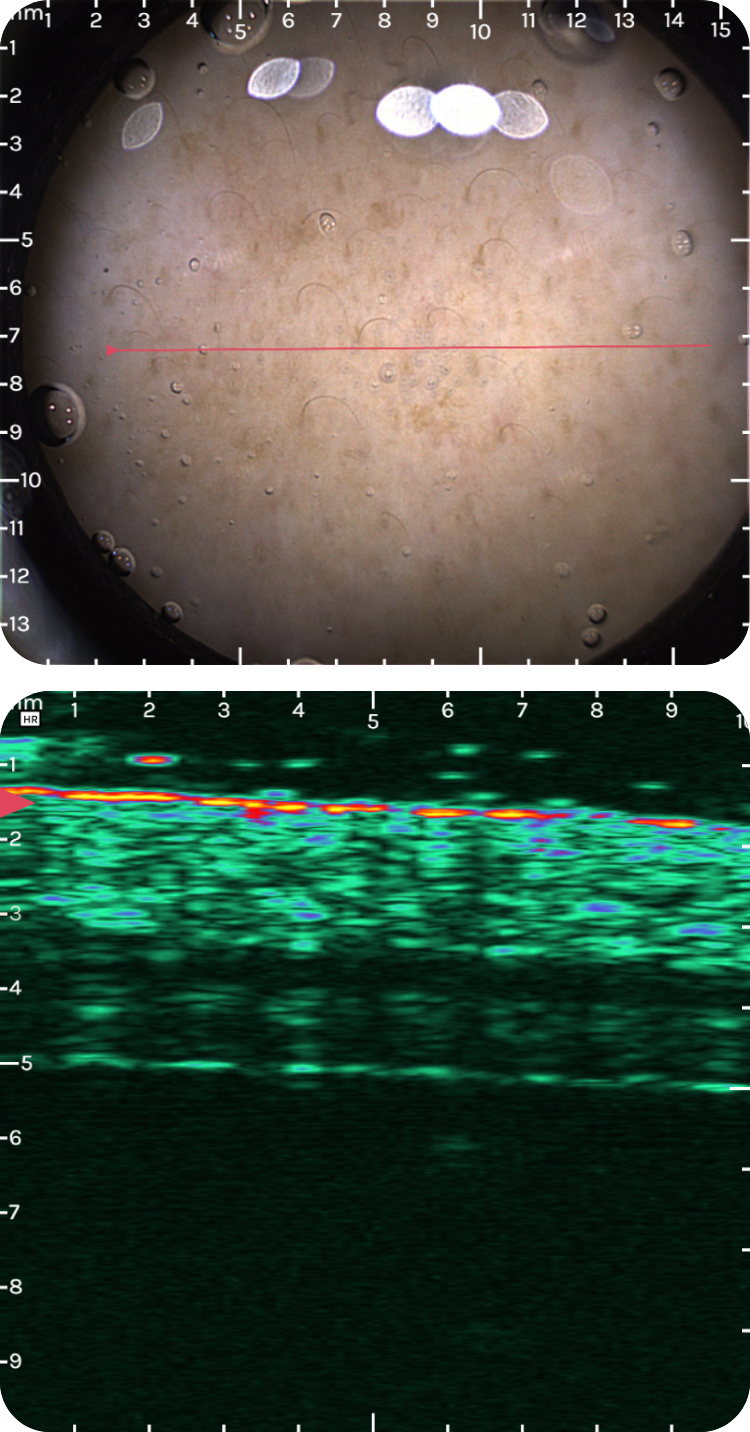
sdfsdfs

sf sdfsdfsdfs sdf sdf sdfsdfsd

sdfsdfsdfsdf

sdfsdfsdfsdf
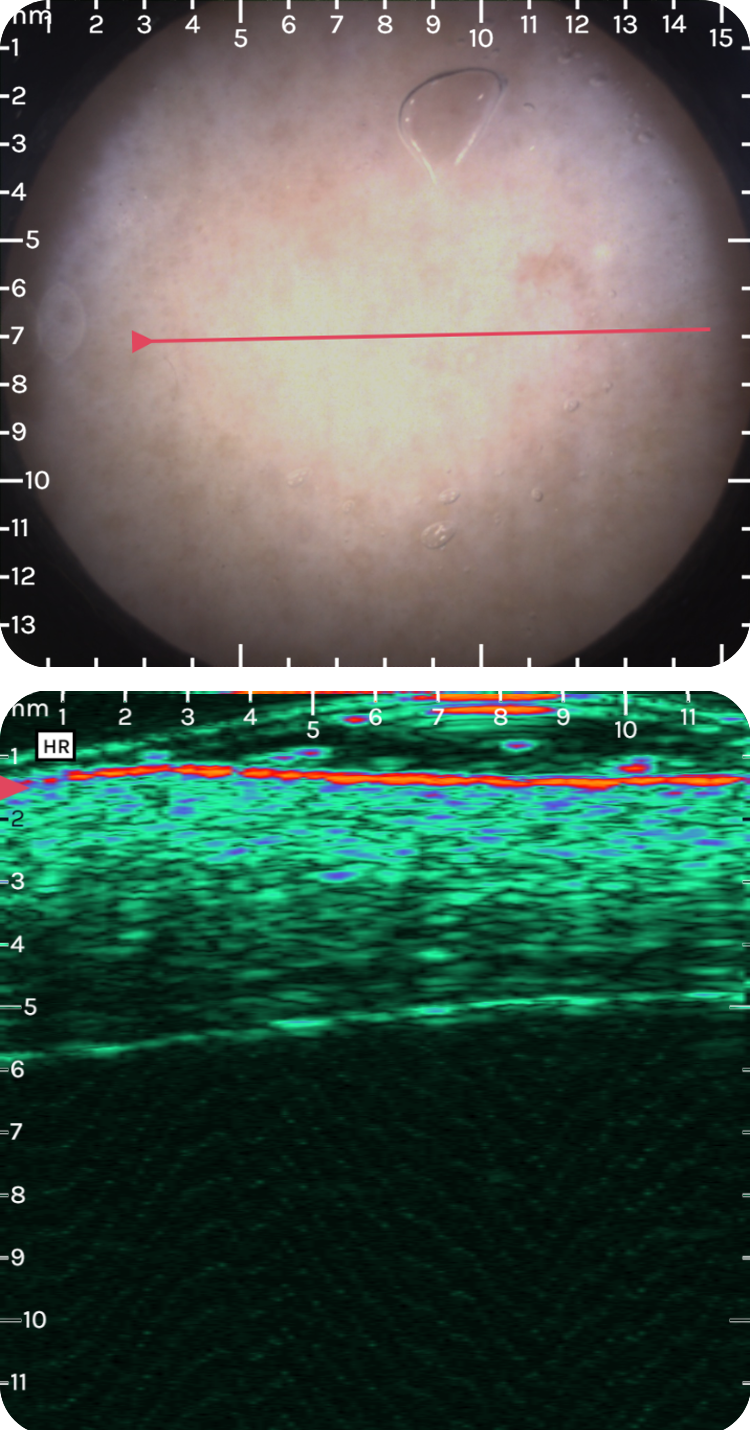
sdfsdfsdf
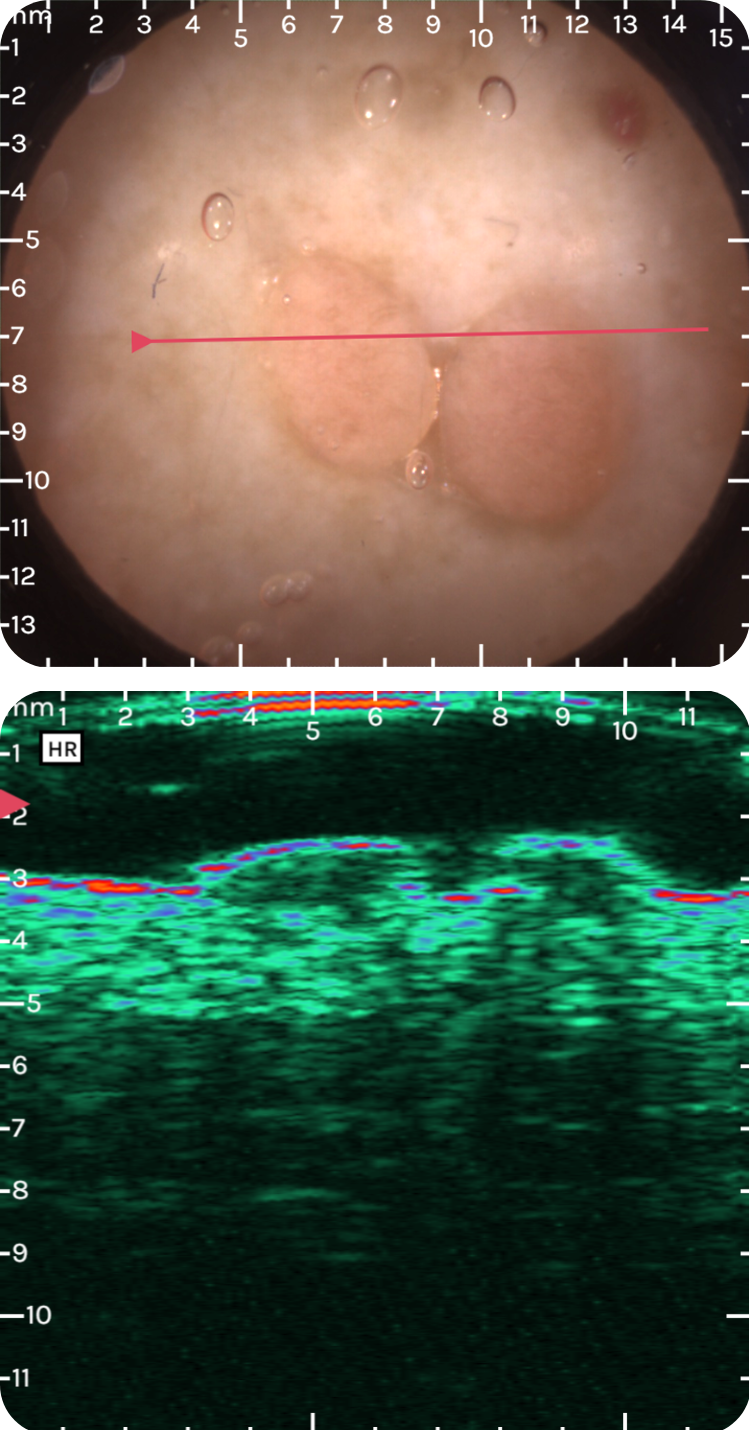
sdfsdfsdfsd
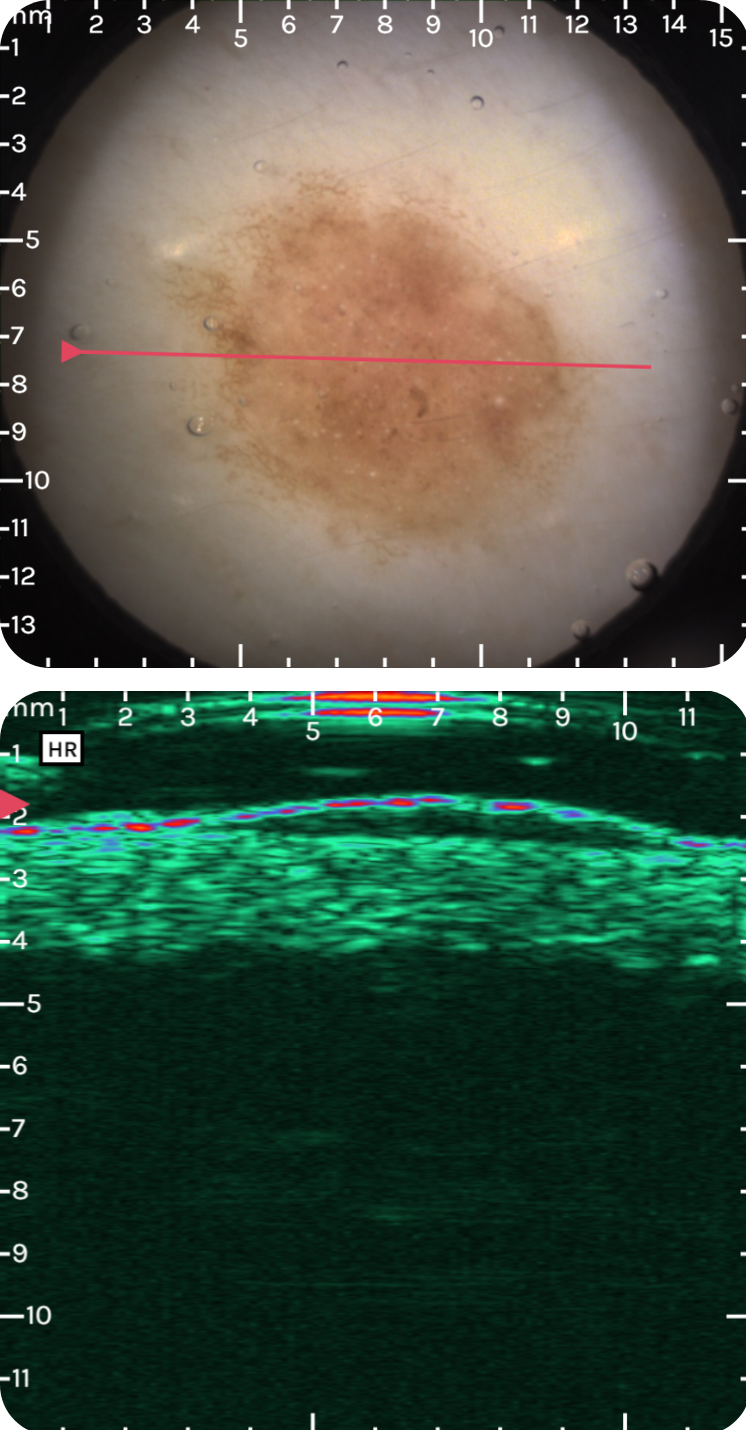
sdfsdfsdfsdf
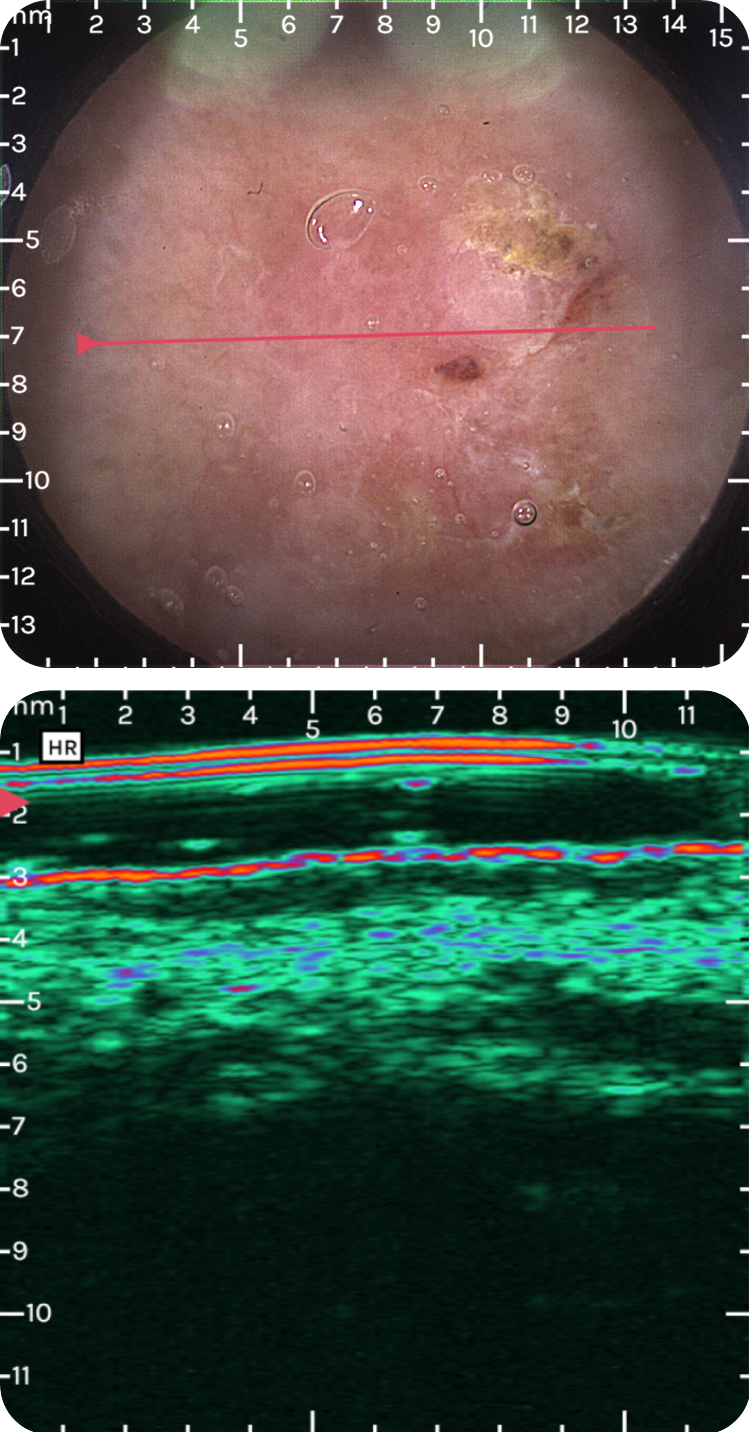
sdfsdfsdf
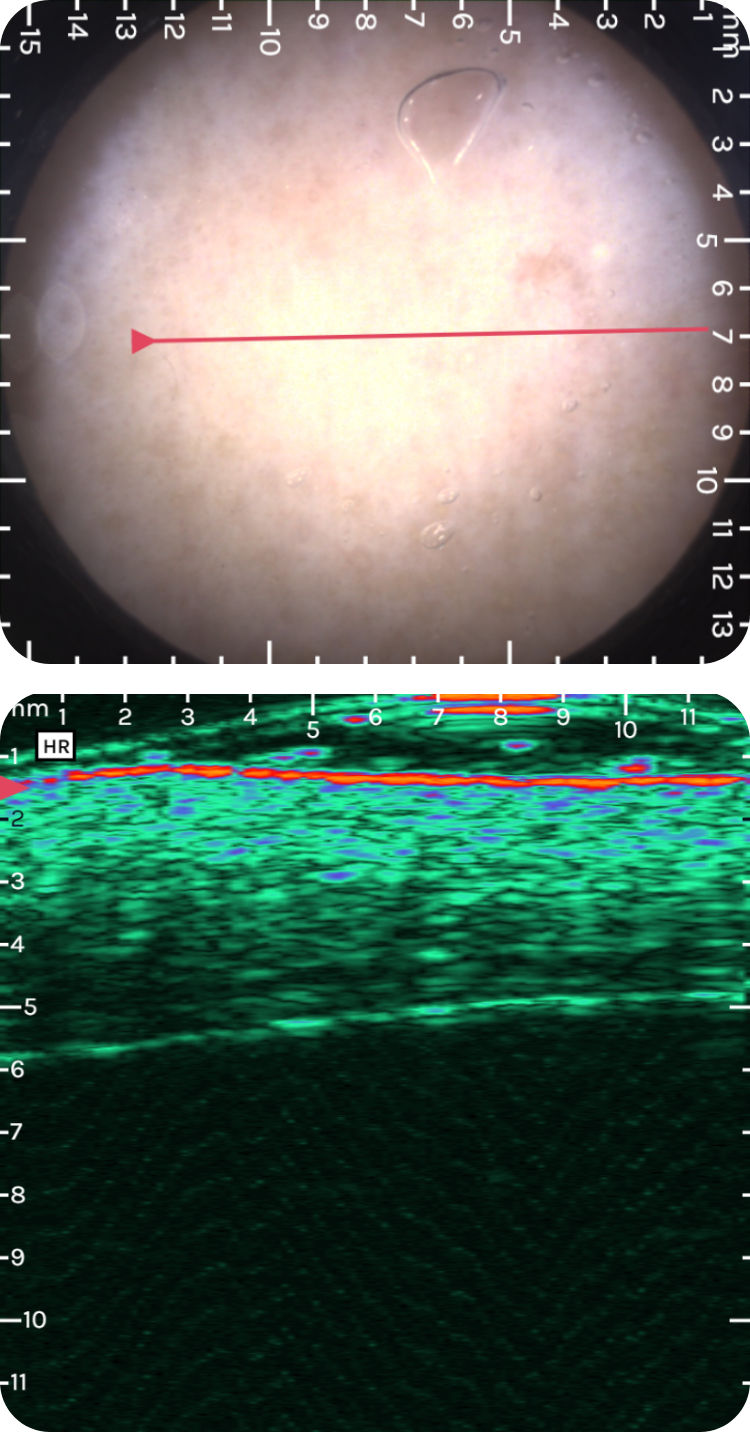
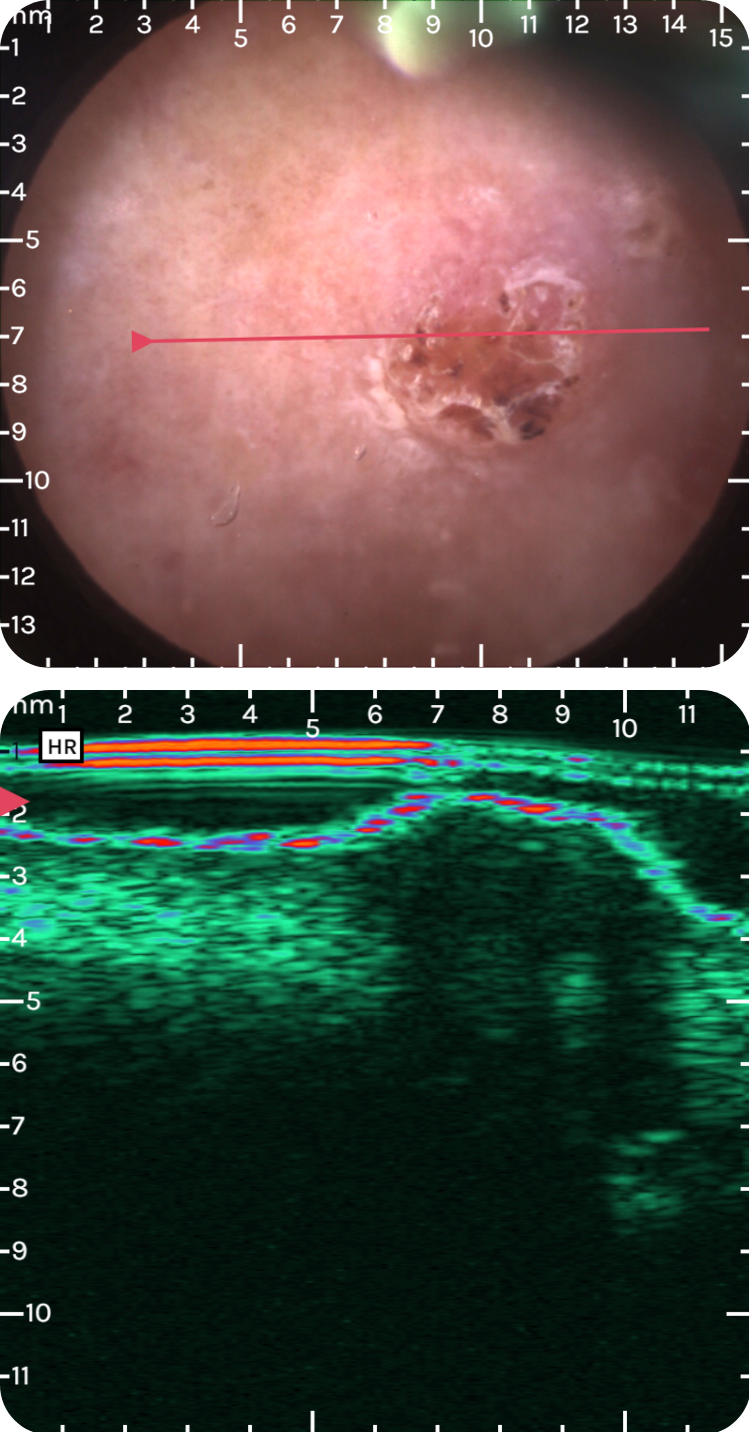
sdfsdfsdf
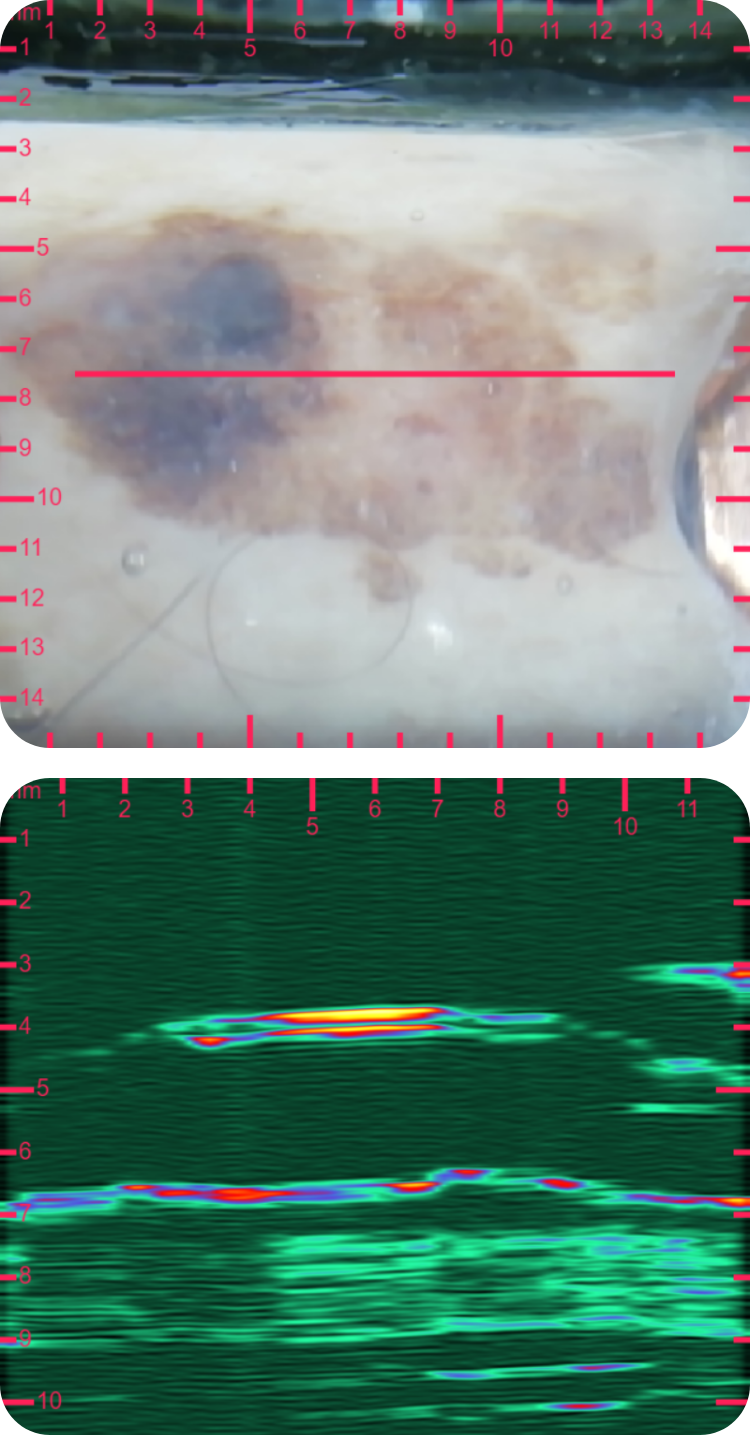

sdfsdfsdf

sdfsdfsdf
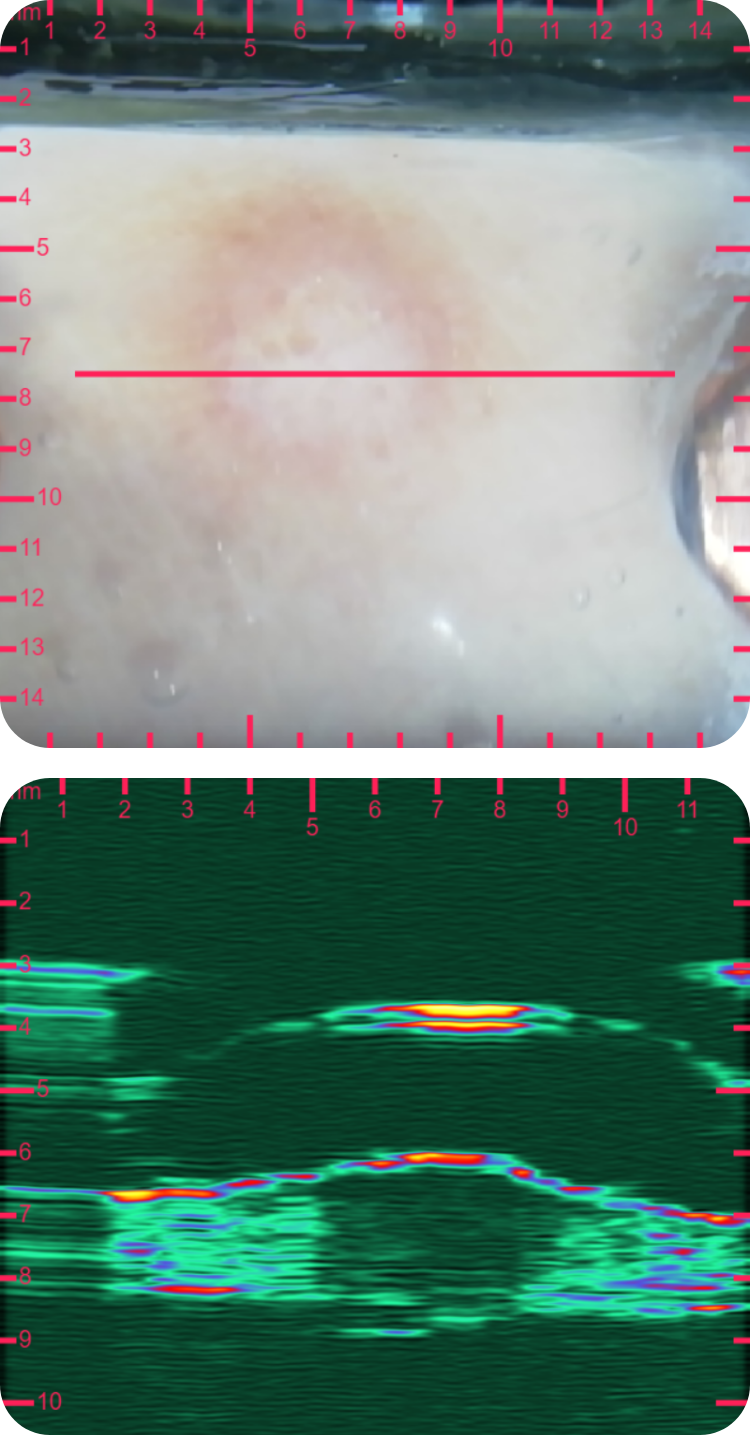
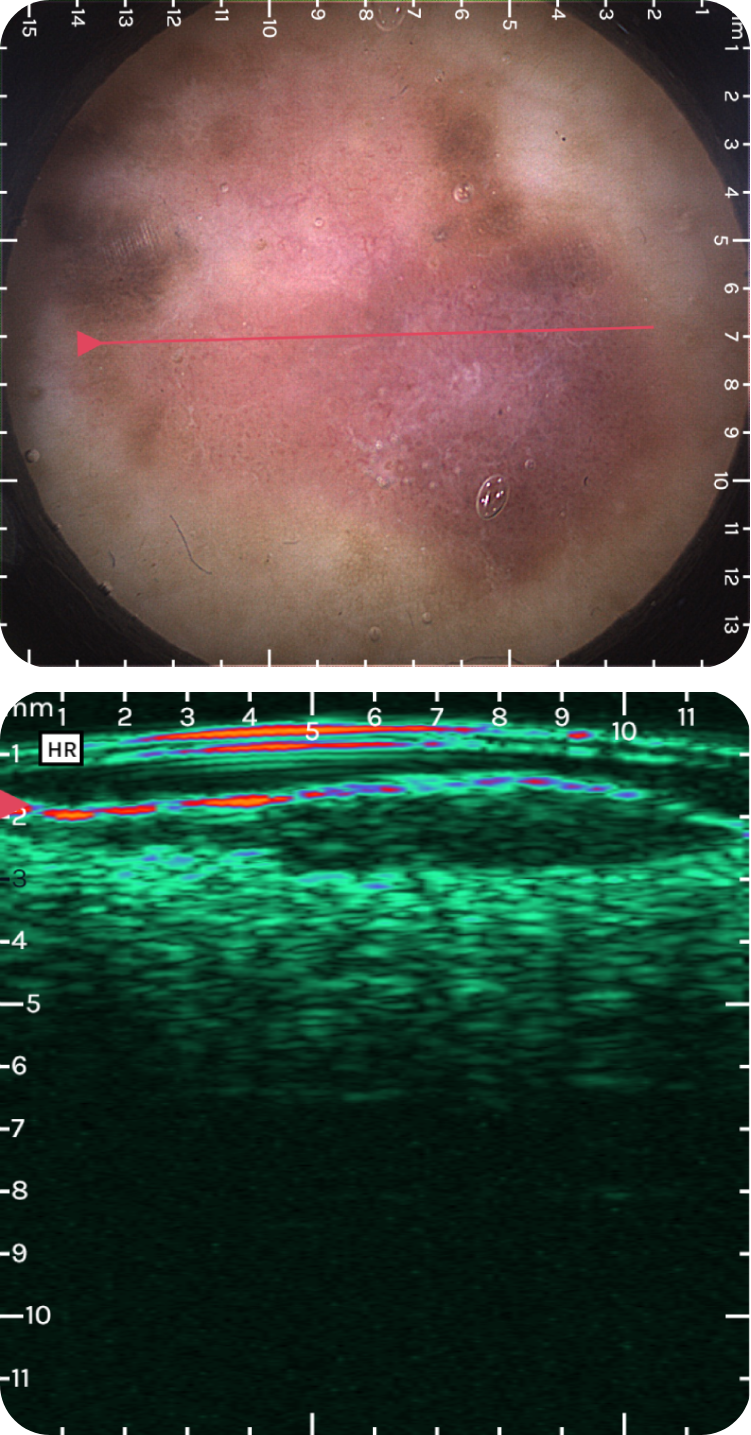

sdfsdfsdf
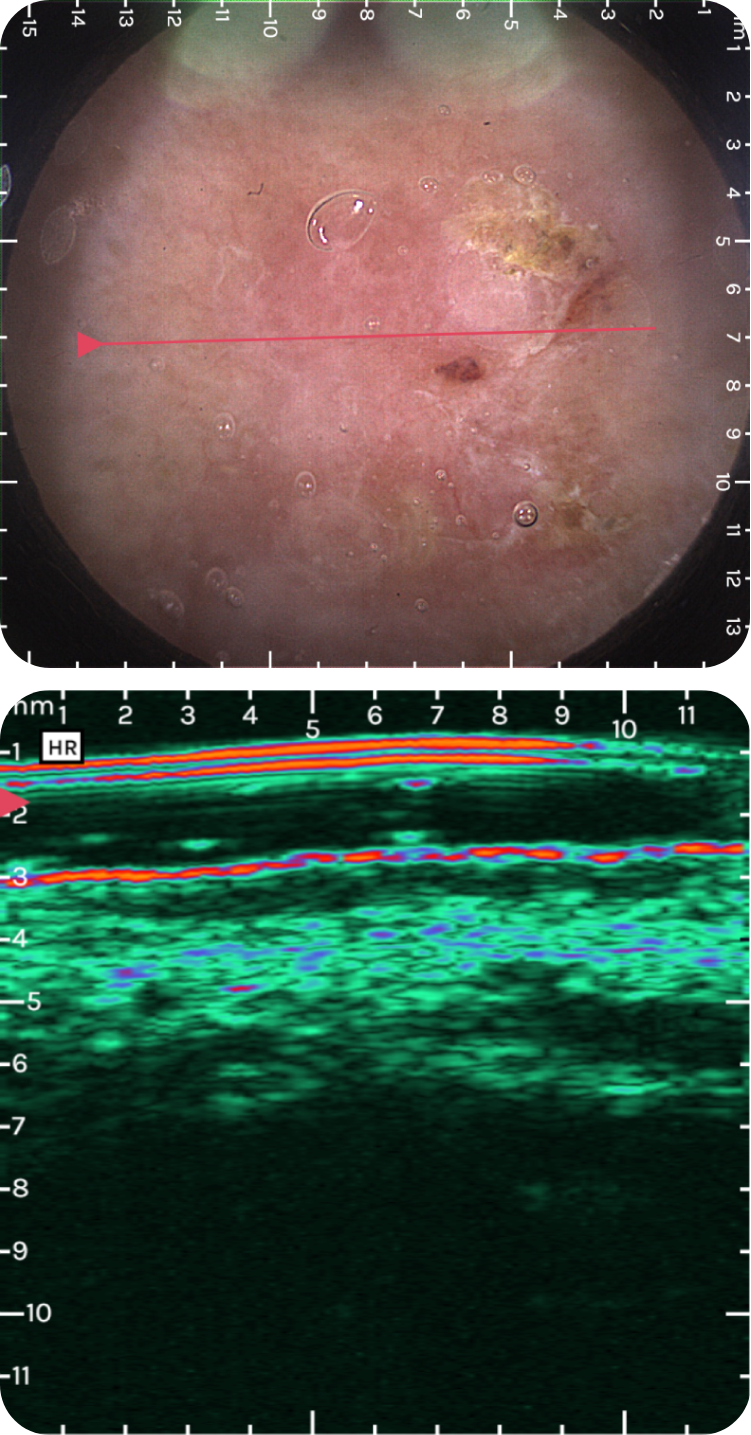

sdfsdfsdf
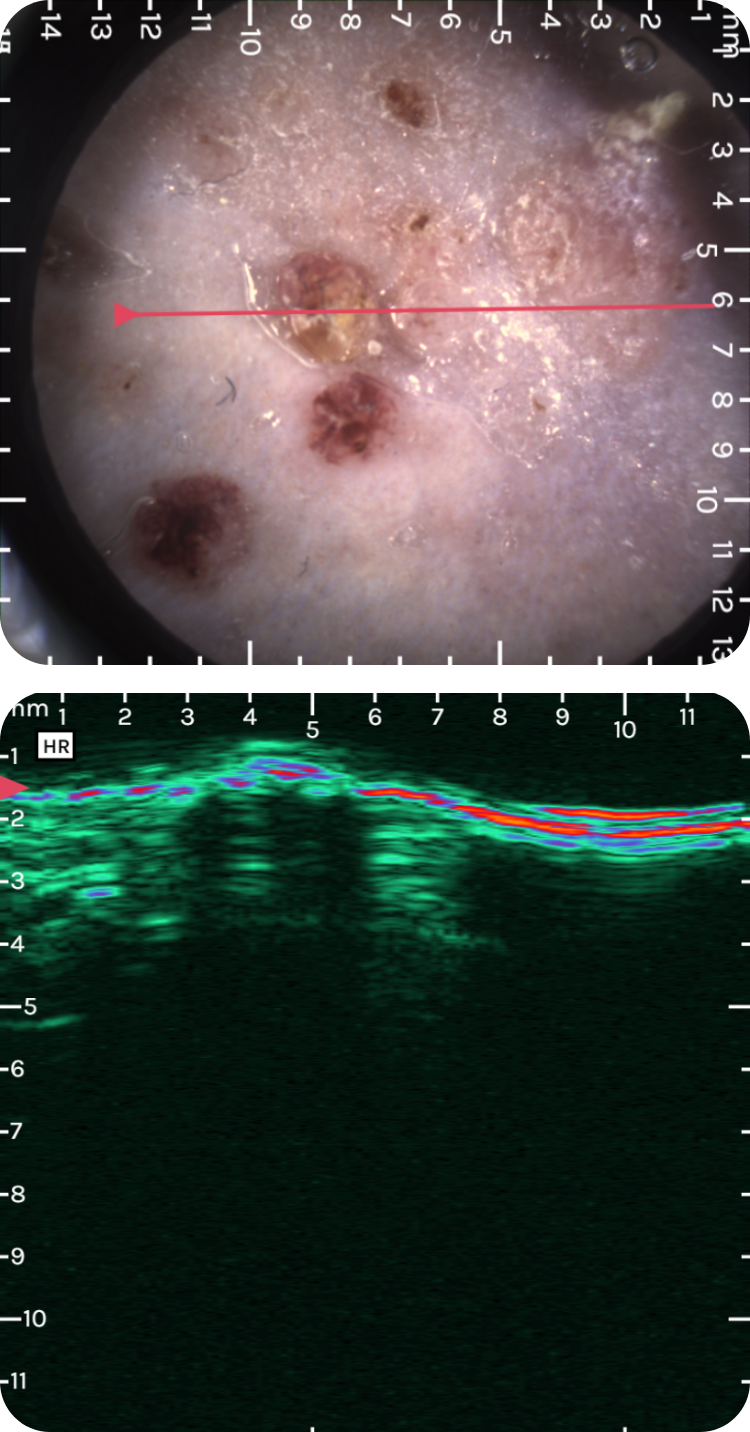
sdfsdfsdf
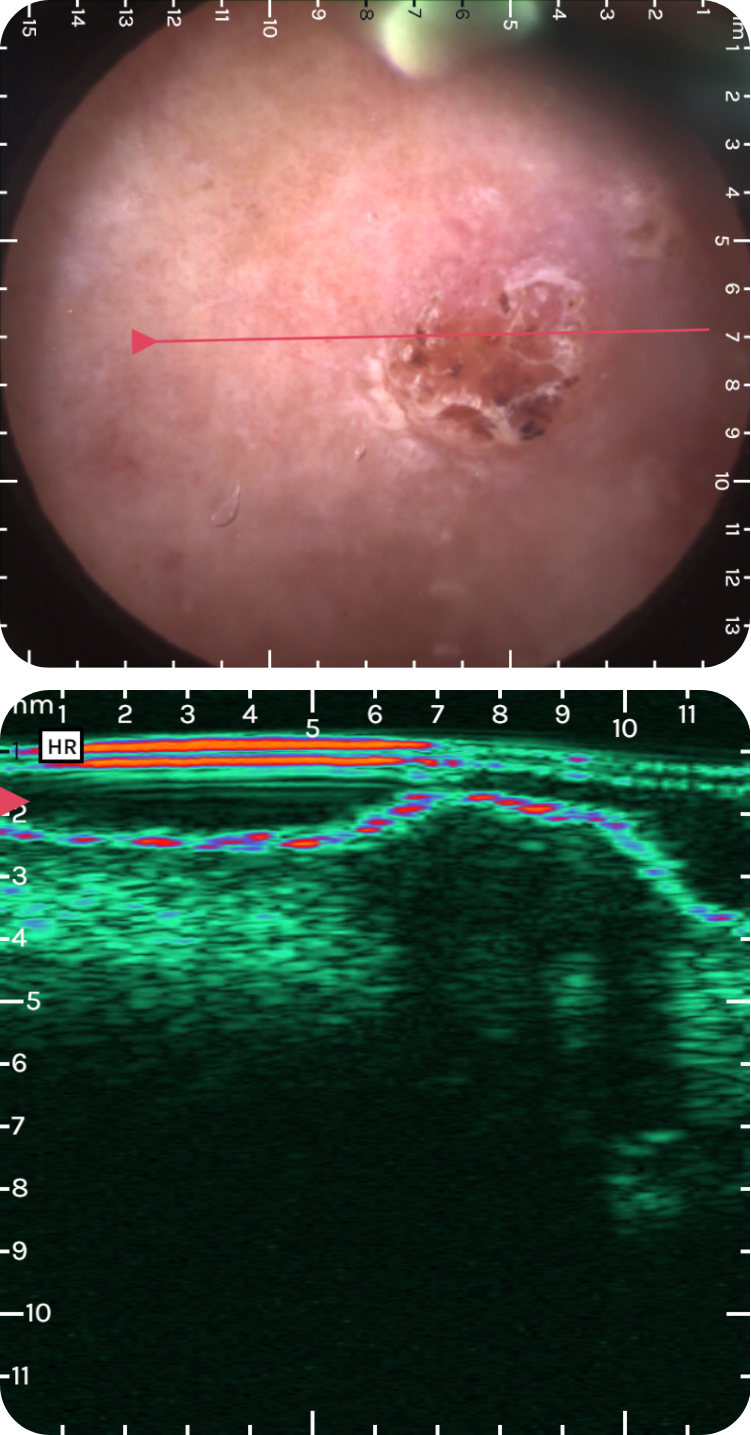
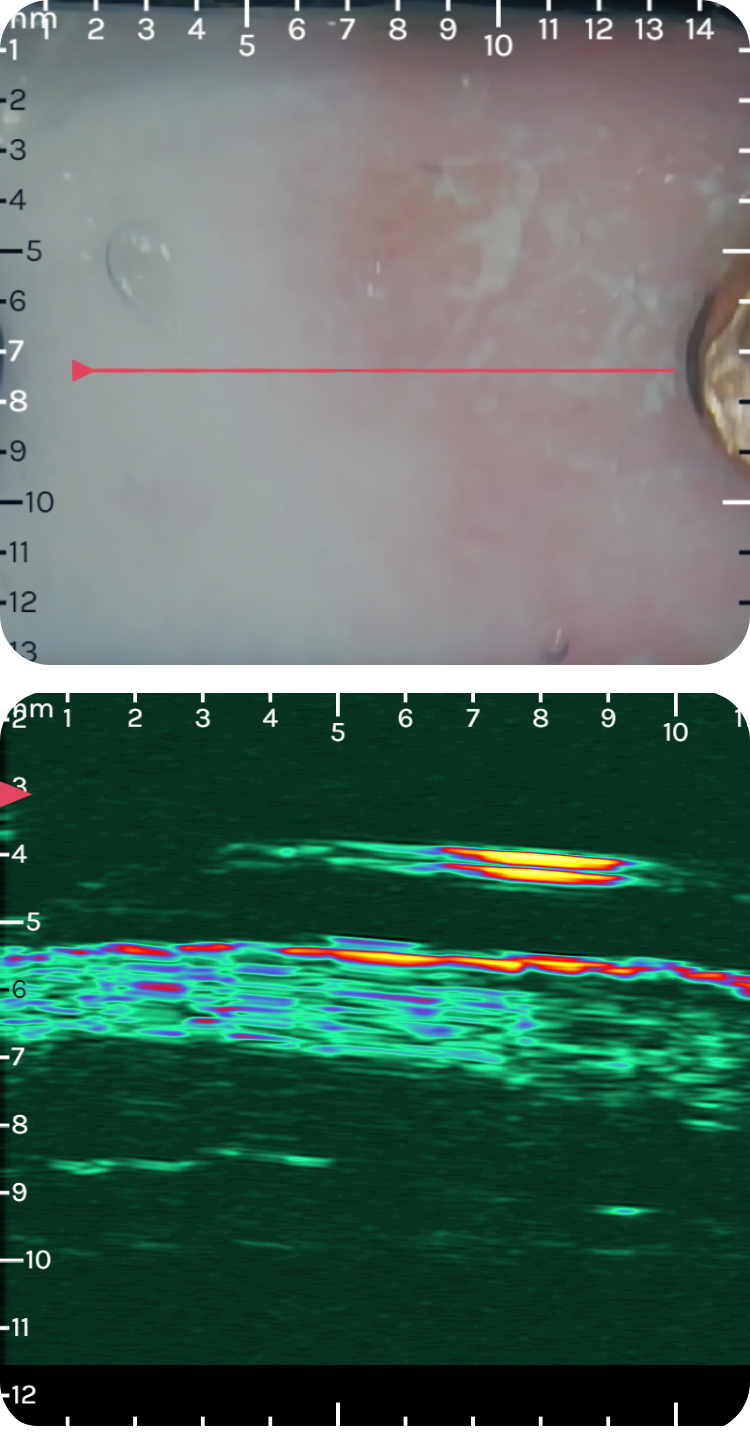
dsfsdfsdf